Pure Element Only One Type of Atom Present
The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes. Atomic number The number of protons in an atom.

Identify The Elements Present In Each Of The Following Compounds And The Number Of Each Element Atom Present Common Salt Nacl Nitric Acid Ppt Video Online Download
The atomic weight values may be different from one periodic table to another because its a calculated number based on the weighted average of the natural isotopes of an element.

. Atomic number The number of protons in an atom. Single type of atom is called an element. It is represented as 1 1 H.
It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compoundsOxygen is Earths most abundant element and after hydrogen and helium it is the third-most abundant element in the. Is it possible for the atom of an element to have one electron one proton and no neutron. By their chemical composition pure substances can be divided into two types elements and compounds.
The 3 subatomic particles that form a part of this tiny universe are neutrons protons and electrons. Note if you have a sample of a. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons.
These blocks are named for the characteristic spectra they produce. Yes hydrogen has one electron one proton and no neutron. Block Elements are organised into blocks by the orbital type in which the outer electrons are found.
Short Answer Type questions. An atom is the smallest particle of an element yet within it there is a universe of its own. The atomic number of each element increases by one reading from left to right.
Sharp s principal p diffuse d and fundamental f. Oxygen is the chemical element with the symbol O and atomic number 8. Elements An element is a pure substance that contains a single kind of atom.
If a new supply of an element is discovered the isotope ratio may be different from what scientists previously believed. These blocks are named for the characteristic spectra they produce. A pure substance that is made of only one type of molecule is called a compound.
Pure substances are made of only one type of atom or molecule. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. Mixing two pure substances results in a.
Write any two observations which support the fact that atoms are divisible. Sharp s principal p diffuse d and fundamental f. Gold silver iron and aluminium are pure substances to name a few.
Then the number may change. According to the law of definite proportions a pure chemical compound broken down into elements always contains elements of a fixed ratio independent of where and how it was created. The substances that contain only one type of particle and are free from any mixture are known as pure substances.
The neutrons have no net charge and are in the center of the atom along with positively charged protons. The atomic number of each element increases by one reading from left to right. The neutrons and the protons together form the nucleus of the atom.
If so name the element. Hydrogen gas and pure iron are examples of pure substances. Hydrogen consists of hydrogen atoms only while iron consists of only iron atoms.
For example pure water will always. A pure substance refers to an element or a compound that has no component of another compound or element.
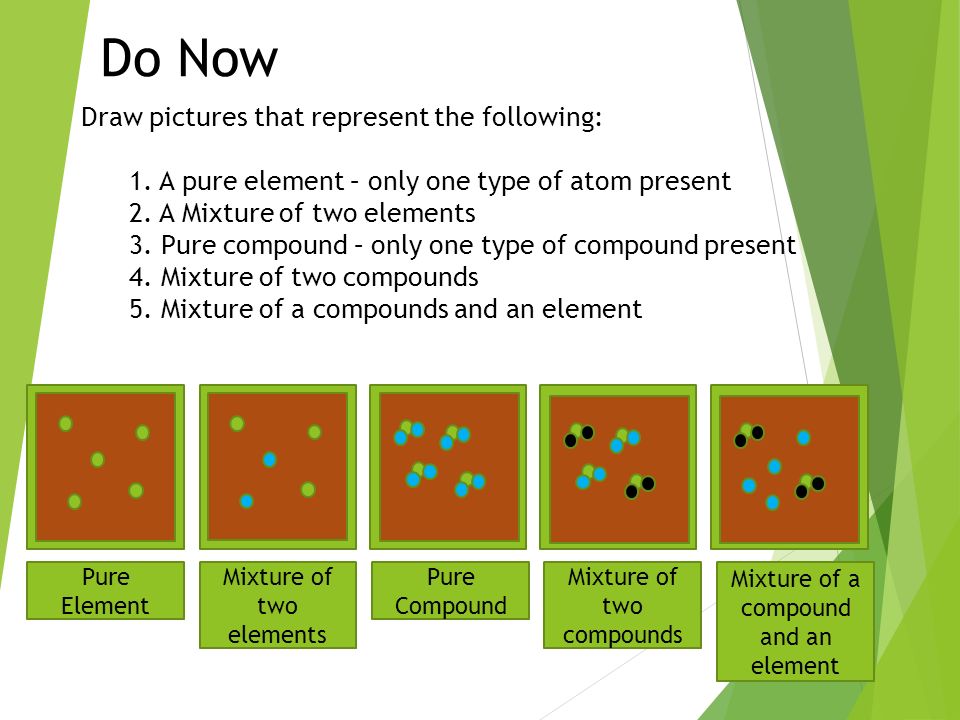
Draw Pictures That Represent The Following 1 A Pure Element Only One Type Of Atom Present 2 A Mixture Of Two Elements 3 Pure Compound Only One Ppt Download

Gcse Edexcel Chemistry Key Concepts In Chemistry Covalent Bonding Complete Revision Summary Notes Vi Covalent Bonding Gcse Chemistry Chemistry
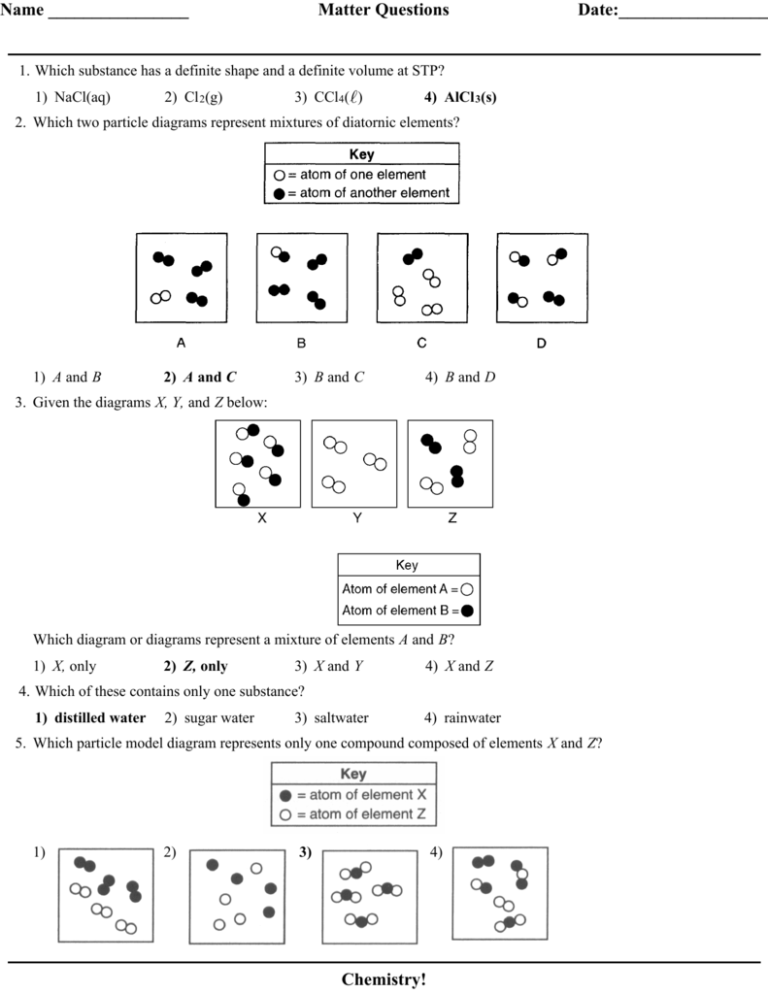
No comments for "Pure Element Only One Type of Atom Present"
Post a Comment